Alnylam Pharmaceuticals is responsible for the funding and content of this website. The site is intended for Healthcare Professionals in Europe, Middle East and Africa. For disease awareness purposes only.
Alnylam Pharmaceuticals is responsible for the funding and content of this website. The site is intended for Healthcare Professionals in Europe, Middle East and Africa. For disease awareness purposes only.
If PH1 is suspected, some common methods seen in clinical practice to help test for the disease are listed below.
This testing information is provided for educational purposes only and is not intended to replace the independent medical judgement of any healthcare professional.
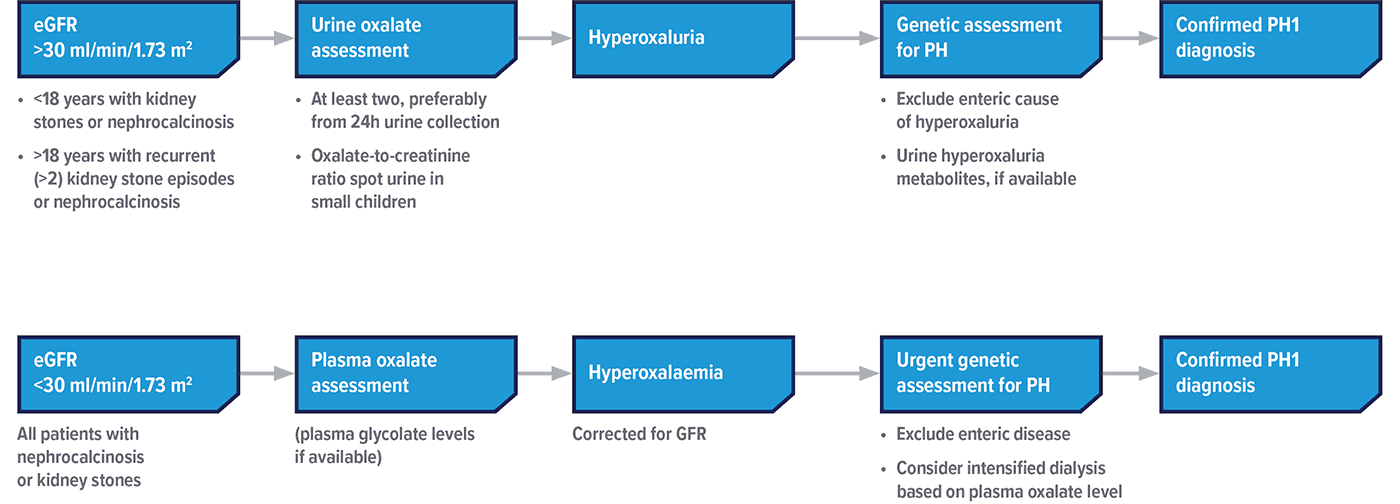
Adapted from Groothoff JW, et al. Nat Rev Nephrol. 2023.
ERKNet and OxalEurope 2023 clinical practice recommendations advise that all patients with suspected PH1 undergo genetic testing7
eGFR, estimated glomerular filtration rate.
Centres de Référence des maladies rénales rares (Filiére ORKiD):
MARHEA – Paris
01 44 49 43 82
Néphrogones – Lyon
04 27 85 61 28
SoRare – Toulouse
05 34 55 86 64 (enfants) ‐ 05 61 32 32 93 (adultes)
Genilam

Diagnostic tests can be requested free of charge by a healthcare professional. In case of suspicion of hyperoxaluria, the healthcare professional can request the test by writing an email to this address:
proyecto.exhplora@vallhebron.cat
Commissioned and paid for by the National Health Service (NHS) via Trust mapped Genomic Laboratory Hub.
Primary Hyperoxaluria Genetics
R257 Unexplained young onset end-stage renal disease8
PH1-INTR-00022 | January 2025

ThinkPH1 is a trademark of Alnylam Pharmaceuticals, Inc.
©2025 Developed and produced by Alnylam Switzerland GmbH. All rights reserved.
PH1-INTR-00025
January 2025
Alnylam Pharmaceuticals is responsible for the funding and content of this website. The site is intended for Healthcare Professionals in Europe, Middle East and Africa. For disease awareness purposes only.
By accessing the website, you confirm to be a Healthcare Professional from Europe, Middle East, or Africa:
If you are not a Healthcare Professional, please access the LivingwithPH1.eu website here.