Alnylam Pharmaceuticals is responsible for the funding and content of this website. The site is intended for Healthcare Professionals in Europe, Middle East and Africa. For disease awareness purposes only.
Alnylam Pharmaceuticals is responsible for the funding and content of this website. The site is intended for Healthcare Professionals in Europe, Middle East and Africa. For disease awareness purposes only.
WHAT MORE MAY BE
Recurrent kidney stones in an adult or any kidney stone in a child may be early signs of a metabolic stone disease with potentially devastating consequences, such as primary hyperoxaluria type 1 (PH1).1–4
Any stone or a family history of stones may be red flags.1,3–5
Recurrent and/or unusual stones* may be red flags.1,6–9
A 24-hour urine test can be used to help detect metabolic stone disease.10–12
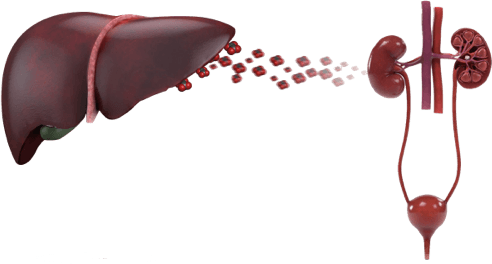
See how genetic testing plays an important role in a PH1 diagnosis.3,11
References: 1. Ferraro PM, D’Addessi A, Gambaro G. Nephrol Dial Transplant. 2013;28(4):811–820. 2. Hoppe B. Nat Rev Nephrol. 2012;8(8):467–475. 3. Milliner DS, Harris PC, Sas DJ, et al. Primary hyperoxaluria type 1. GeneReviews® [Internet]. Updated 15 August 2024. Accessed January 2025. https://www.ncbi.nlm.nih.gov/books/NBK1283/ 4. Cochat P, Rumsby G. N Engl J Med. 2013;369(7):649–658. 5. Hoppe B, Kemper MJ. Pediatr Nephrol. 2010;25(3):403–413. 6. Jendeberg J, Geijer H, Alshamari M, et al. Eur Radiol. 2017;27(11):4775–4785. 7. Carrasco A Jr, Granberg CF, Gettman MT, et al. Urology. 2015;85(3):522–526. 8. Hoppe B, Beck BB, Milliner DS. Kidney Int. 2009;75(12):1264–1271. 9. Leumann E, Hoppe B. J Am Soc Nephrol. 2001;12(9):1986–1993. 10. Ben-Shalom E, Frishberg Y. Pediatr Nephrol. 2015;30(10):1781–1791. 11. Cochat P, Hulton SA, Acquaviva C, et al. Nephrol Dial Transplant. 2012;27(5):1729–1736. 12. Groothoff JW, Metry E, Deesker L, et al. Nat Rev Nephrol. 2023;19(3):194–211. 13. Harambat J, Fargue S, Acquaviva C, et al. Kidney Int. 2010;77(5):443–449. 14. van der Hoeven SM, van Woerden CS, Groothoff JW. Nephrol Dial Transplant. 2012;27(10):3855–3862. 15. Hulton SA. Int J Surg. 2016;36(Pt D):649–654. 16. Hopp K, Cogal AG, Bergstralh EJ, et al. J Am Soc Nephrol. 2015;26(10):2559–2570. 17. van Woerden CS, Groothoff JW, Wanders RJA, et al. Nephrol Dial Transplant. 2003;18(2):273–279. 18. Mandrile G, van Woerden CS, Berchialla P, et al; for OxalEurope Consortium. Kidney Int. 2014;86(6):1197–1204. 19. Lieske JC, Monico CG, Holmes WS, et al. Am J Nephrol. 2005;25(3):290–296. 20. Soliman NA, Nabhan MM, Abdelrahman SM, et al. Nephrol Ther. 2017;13(3):176–182.
PH1-INTR-00019 | January 2025

ThinkPH1 is a trademark of Alnylam Pharmaceuticals, Inc.
©2025 Developed and produced by Alnylam Switzerland GmbH. All rights reserved.
PH1-INTR-00025
January 2025
Alnylam Pharmaceuticals is responsible for the funding and content of this website. The site is intended for Healthcare Professionals in Europe, Middle East and Africa. For disease awareness purposes only.
By accessing the website, you confirm to be a Healthcare Professional from Europe, Middle East, or Africa:
If you are not a Healthcare Professional, please access the LivingwithPH1.eu website here.